Abstract
Background: ITP, characterized by isolated platelet reduction, is classified as persistent within 3-<12 months (mo) of diagnosis and chronic (cITP) when it continues for ≥12 mo. Theoral thrombopoietin-receptor agonist (TPO-RA), EPAG, is approved for the treatment of previously treated (eg corticosteroids, immunoglobulins) cITP patients (pts). Very few analyses have evaluated EPAG treatment specifically in subgroups of persistent ITP pts. To gain better insight into EPAG effects on platelet counts, and long-term safety, in persistent ITP, a subanalysis of a Phase IV, open-label safety study of EPAG in adults with persistent/cITP (Brynes et al. Acta Haematol 2017;137:66-72) was performed.
Aims: To evaluate the effects of EPAG on platelet counts, and long-term safety, during 2 yrs' treatment, in persistent or cITP pts.
Methods: Adults ≥18 yrs old diagnosed with persistent ITP (3-<12 mo) or cITP (≥12 mo) were enrolled in this 2-yr, longitudinal, prospective study. Any prior treatment with EPAG or romiplostim must have been completed ≥6 mo before screening. Pts treated with any other TPO-RA, or with baseline (BL) marrow fibrosis (MF) reticulin grade MF-3, were not eligible. All pts started EPAG at 50 mg/day (25 mg for East Asian pts), adjusted to a lower dose or maximum 75 mg dose as required to maintain platelet counts within the clinically indicated range. This analysis evaluated effects of EPAG on platelet counts and MF, and safety during 2 yrs' treatment in persistent or cITP pts.
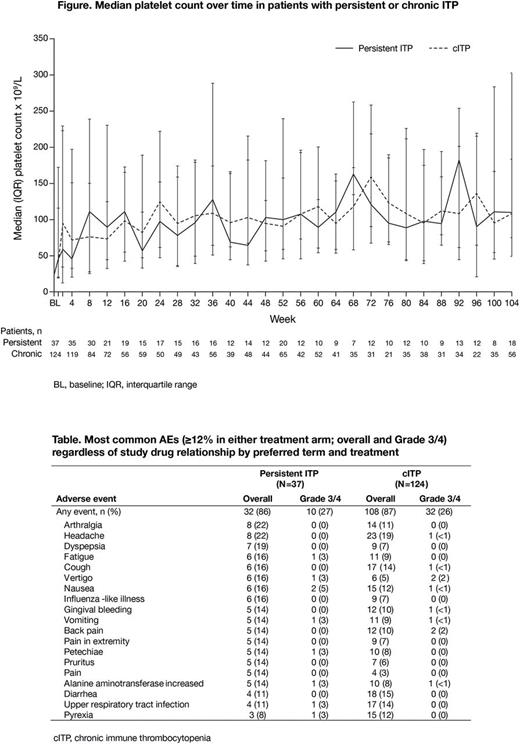
Results: At BL, 37/162 (23%; mean ± SD age 44 ± 16 yrs; 57% female) and 124 (77%; mean ± SD age 43 ± 16 yrs; 66% female) pts had persistent or cITP, respectively. One pt (1%) had ITP diagnosis duration <3 mo. Among persistent ITP pts, 8% were splenectomized, 51% had platelets <30×109/L and 100% had no prior TPO-RA exposure; of those with cITP, 27% were splenectomized, 60% had platelet <30×109/L and 90% had no prior TPO-RA exposure. 13 persistent ITP pts (35%) and 31 cITP pts (25%) withdrew early from the study, most commonly because of AEs (n=8, 22% and n=13, 10%, respectively) and lack of efficacy (n=4, 11% and n=7, 6%). Median exposure duration was 2.0 yrs (range 31 days to 2.1 yrs) and 2.0 yrs (range 21 days to 2.2 yrs); mean daily dose was 48.8 (range 11-75) mg/day and 48.8 (range 5-75) mg/day. Median platelet counts in all pts increased to ≥50×109/L within 1 wk in both groups (Figure). Overall, 31 (84%) persistent ITP and 109 (88%) cITP pts achieved a platelet count ≥50×109/L without rescue therapy; 19 (51%) and 62 (50%) maintained platelet counts continuously ≥50×109/L for ≥28 wks. In pts with BL platelets <50×109/L who achieved platelet counts ≥50×109/L (on treatment, no rescue therapy; n=25 persistent ITP, n=99 cITP), median (95% CI) time to platelets ≥50×109/L was 4.0 (1.3-14.4) wks in persistent ITP and 2.1 (1.4-2.1) wks in cITP pts. Sixteen (43%) persistent and 50 (40%) cITP pts received rescue therapy, most frequently a new ITP medication (n=9 [24%] and n=34 [27%], respectively).
Compared with BL, 17/18 persistent ITP pts (94%) remained MF-0 at 2 yrs, 1 (6%) had a 1-grade increase; in cITP pts, 62/75 (83%) remained MF-0, 8 (11%) had a 1-grade increase, 2 had a 1-grade decrease and 1 (1%) remained MF-1. No pts had symptoms or abnormalities typical of MF.
AEs were reported in 32 (86%) persistent ITP pts and in 108 (87%) cITP pts (Table). Serious AEs occurred in 12 (32%) persistent ITP pts, most frequently (≥5%) nausea (n=2, 5%) and fatigue (n=2, 5%), and in 29 (23%) cITP pts, most frequently (≥2%) gastrointestinal hemorrhage, gingival bleeding, tooth abscess, cerebral hemorrhage, lethargy, thrombocytopenia and menorrhagia (all n=2, 2%). No pt with persistent ITP died while on treatment (+ 1 day), and 3 (2%) cITP pts died (cerebral hemorrhage, n=2 [platelets <5 and 7×109/L, not considered drug-related] and acute respiratory distress syndrome, n=1).
Summary/conclusions: The effects of EPAG on platelet counts in persistent ITP pts were consistent with that seen in the cITP population, with sustained platelet increases. Overall AE rates were similar in both subgroups and AEs reported were consistent with the known EPAG safety profile or underlying disease. While there appeared to be an increase (>10% difference) in some AEs (vertigo, dyspepsia, arthralgia) in persistent ITP pts, this could be attributed to the limited pt number in this subgroup. These results are encouraging; outcomes following EPAG treatment in persistent ITP are consistent with reports in cITP.
Wong: Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Myers Squibb: Research Funding; GlaxoSmithKline: Research Funding; Johnson & Johnson: Research Funding; Merck Sharp & Dohme: Research Funding; Pfizer: Research Funding; Roche: Research Funding; Biogen-Idec: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer: Consultancy, Research Funding. Brynes: Novartis: Research Funding. Arikan: Novartis: Employment. Maier: Novartis: Employment. Shamsi: Fresenius Germany: Research Funding; Novartis Pharma Pakistan: Consultancy; Roche Diagnostic Pakistan: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal